
It’s not easy to tell what the water temperature just by looking at it.
Say that you are going to brew gyokuro, so you’re aiming for 60ºC (140ºF).
How can you tell when the water reaches that temperature without using a thermometer and looking like a geek (like me)?
The 10ºC rule of thumb
A popular technique to guess the temperature of water consists of boiling water first (100ºC, 212ºF at sea level) and each time that you pour it the temperature drops by 10 degrees celcius.
For gyokuro, for example, first boil water (100ºC, 212ºF). Then pour into the kyusu, so that the water temperature drops to 90ºC (194ºF). Then pour that water into your cup, and now you have 80ºC (176ºF).
The next step is to pour that water into what’s called a yuzamashi (if you don’t have one just use another cup or any other container), that way you just reached 70ºC (158ºF). That is the temperature to brew sencha, by the way.
We need 60ºC (140ºF). To make that happen all we need to do is pour again, this time into the kyusu with the gyokuro leaves inside.
Sounds easy enough, right? Let’s go one step further and actually measure things to see how accurate this method really is.
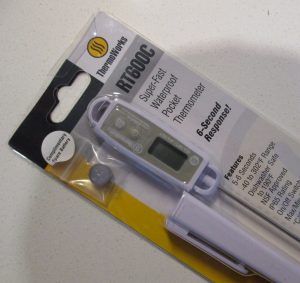 The experiment
The experiment
I used my newly bought digital thermometer to measure the water temperature. It displays both celcius and fahrenheit.
First I boiled water, measured its temperature, poured into a measuring cup and immediately took the temperature again.
Then I did the same thing for two more cups (Japanese yunomi, 湯呑み) and the whole process was repeated 3 times for a better sample.
Each new trial I washed the cups with cool water so as not to alter the results because of warm cups.
When taking the temperature I had the thermometer inside the water, but not touching the surface of the container to have a more accurate reading.
Local conditions
 Before telling you what the results where, let me give you a little background to better understand the data.
Before telling you what the results where, let me give you a little background to better understand the data.
This experiment was conducted in Bogota, Colombia. Bogota has an altitude of 2,625 m (8,612 ft).
Why does this matter? Because the boiling point varies with atmospheric pressure, and at higher altitudes the pressure is less. The lower the pressure, the lower the boiling point.
Water boils at 100ºC (212ºF) at sea level, but at my city it boils at approximately 91.2ºC (196.1ºF)! That’s the reason why boiling an egg takes so long in Bogota, the temperature of boiling water is too low to cook the egg quickly.
For those of you in Denver, Colorado, the boiling point of water is 94.0ºC (201.2ºF). Meanwhile in Tokyo, my favorite city in the world, water boils at 99.8ºC (206.2ºF).
If you want to do your own boiling point calculations, try this online calculator. You just have to find out the altitude of your city.
Also, the room temperature was 20ºC (68ºF) and there is very low humidity in this city. I’m sure there are lots of variables that affect the cooling rate of water, therefore this experiment is far from being rigorous. However, I just want to give you a general idea of how much does water cool when pouring.
As a bonus, I also took temperatures from the last cup to see how much did it cool by just letting it be idle for a minute.
Results
 The following measurements where taken in degrees Celsius.
The following measurements where taken in degrees Celsius.
The first measurement is taken of water boiling in a pot, and then the temperature immediately after pouring into a cup.
Two more pourings from cup to cup were also measured.
First trial
| Description | Temperature | Difference |
|---|---|---|
| Boiling in pot | 91.5 | |
| First pour | 88.1 | 3.4 |
| Second pour | 81.6 | 6.5 |
| Third pour | 72.5 | 9.1 |
Second trial
| Description | Temperature | Difference |
|---|---|---|
| Boiling in pot | 91.4 | |
| First pour | 88.5 | 2.9 |
| Second pour | 82.6 | 5.9 |
| Third pour | 73.9 | 8.7 |
Third trial
| Description | Temperature | Difference |
|---|---|---|
| Boiling in pot | 91.5 | |
| First pour | 87.7 | 3.8 |
| Second pour | 80.5 | 7.2 |
| Third pour | 72.6 | 7.9 |
The average drop in temperature was:
From pot into first cup, 3.4ºC
From first cup to second cup, 6.5ºC
From second cup to third cup, 8.5ºC
Cooling of water after 1 minute in cup
| Trial | Initial | Final | Difference |
|---|---|---|---|
| 1 | 62.9 | 60.0 | 2.9 |
| 2 | 67.0 | 64.1 | 2.9 |
| 3 | 66.2 | 63.7 | 2.5 |
Conclusions
The water seemed to cool better at later pourings in the experiment. I Probably should have used the same ceramic cup instead of a plastic one (more insulation) for the first pouring, but it would have been harder to pour the boiling water from the pot into such a small cup.
Anyway, in my case I couldn’t achieve the 10ºC just by pouring immediately. This is where the other part of the experiment comes in: by waiting for some time, the water keeps cooling inside the cup. I should wait about a minute after each pouring for a better cooling.
What I learned from this experiment is that trying to achieve a specific temperature for brewing is no simple task. Nevertheless, at the very least it’s important to know the local boiling point so as to have a good starting point.
If you pause between pourings you may get closer to the 10ºC cooling, so the rule of thumb may give you a good approximation after all.

December 3, 2020
Hi Ricardo, I have found this experiment very interesting, but it doesn’t really recognise the basic question which led me to it via Google – how much does boiling water cool when poured into a ceramic mug at room temperature (say, 20 degs C)? Of course, mugs vary in size and weight, but the outside of the mug itself gets very hot, so the water must be losing heat. I want my water at about 80 degs C before making green tea. Sometimes I make Bancha, which I believe is Japanese green tea. More often it is Lung Ching (Dragonwell), which is currently my favourite tea to enjoy in the afternoon. So I leave my hot water in the mug for a few minutes and hope it’s about the right temperature, but sometimes forget it and have to warm it up again in the microwave before making the tea! Anyway, this has led me to your website, so I will spend some time looking around it to find out more about your ideas and experiences of Green Tea. I liked Tokyo very much as well, but preferred the Japanese countryside in Hokkaido and on the Nakasendo Highway. Two years ago we were in Peru, not so far from Bogota. The lower boiling point must be good for making green tea! Now my tea is ready to drink.
Thank you.
December 3, 2020
Hi Andy
It’s hard to have an exact answer because there are many variables at play.
For example, I’m using cups with 120 ml of water. If you have more water, it would take longer to cool down.
But anyway it can give you an idea. The most important thing is to do it in a way that you find the taste to be enjoyable.
June 6, 2021
I live at 500 feet ASL. Roughly. Water at 29.92 inches of mercury is about 100c for my purpose i consider my boiled water to be 100. It will only ever vary a degree or two at most.
5 -6 minutes for that to cool to a little over 70c which is good for my sencha.
At your altitude, I’d say it’s probably about 3-4 minutes wait but I’m only guessing based on what I assume is a lower pressure in general. (Just checked your altimeter at your airport and you’re showing 30.33 inches mercury pressure so maybe it’s not generally lower!)
Either way, I’d say 3-4 minutes and check with a thermometer. Once you figure out how long it takes in your kitchen in general you can just set a timer.
Hope this helps.
June 9, 2021
Thank you Brandon for the detailed information.
November 20, 2021
Hi Ricardo..Do you know the Japanese words for this method of cooling?Thanks,Ken
November 21, 2021
Hi Ken
They don’t have a specific name for it. In Japanese they just say “cool the water to the appropriate temperature”, for example.
April 19, 2024
Awesome post … I hadn’t specifically heard of this method and also your city comparisons were very interesting. Bout to check out your umami post .. nice writing.
April 21, 2024
Thank you. I hope that you like the post about umami as well.